Protectives and Adsorbents
These are commonly used for the treatment of diarrhea which is caused by bacterial toxins . Chemical poisions and some drugs etc.
Diarrhoea can lead to sihytration and electrolyte imbalance
An antidiaroheal preparation contains protein adsorbents and antibacterial agents
Protective and adsorbents adsorb toxins, bacteria and viruses and provide a protective coating along intestinal mucosa.
Example : KAOLINE
KAOLINE
It is commonly known as china clay . it is a bative hydrated aluminium silicate with variable composition ad its formula is Al2O3·2SiO3·2H2O
It is of 2 types.
Heavy Kaoline
Light Kaoline
Light Kaoline is used as an antidiarroheal agent if is free most of the impurities Kaoline may be obtained from rocks.
Impurities are removed by the recommended method and then it is powdered . It is available as light white powdered free from gritly particle, odourless, almost tasteless and greasy touch.
Practically insoluble in water and in mineral acids.
USES
Light Kaoline belongs to the category of protectives and adsorbents
It is used as an antidiarhoeal agent heavy Kaoline is useful in Topical applications against inflammation.
ANTIMICROBIALS
These are topical agents. Topical agents are used on the body surface and their effect is primarily at the surface to which they are applied.
Topical application of drugs may be accomplished within body cavities that open to the outside .
For e.g:- ORAL, VAGINAL , COLONIC CAVITIES etc.
These are several terms used in describing antimicrobial activity
For e.g :- antiseptic, germicide , disinfectant and sterlisation.
Antiseptic:- Any agent which either Kills or inhibit the growth of microorganism bacterial fungi cidal, Amoebicide.
Germicides :- Any agent which kills micro organism these may be named as bacterial fungi cidal, Amoebicide.
Disinfectant :- Agents which are used on inanimate objects (non-living) germicidal activitiy
For e.g:- used for instruments, equipments rooms etc.
STARLIZATION:- Use of disinfectant or other produce to make an object comlitely free from micro-organism
MECHANISM OF ACTION
Inorganic antimicrobials acts through following mechanism of action:-
Oxidation :- This action involves the reducing group present in most proteins for e.g.:- sulphylryl group (SH) present incytine. E.g.:- Potasium permagnate, Hydrogen peroxide (H2O2) and iodine.
Halogenation :- These agents can cause chlormation of peptide linkage between the A groups comprising the protein molecule. Chlorinated lime.
Protein precipitation :- It involves the interaction of protein with metallic ions. E.g.- AgNO3 , Sulphur and its compounds.
POTASSIUM PERMAGNATETE (KMnO4)
Preparation :- It may be prepared by KOH heating MnO2 in presence of air or oxidizing agents like __ nitrate or . Chlorate
KOH + MnO2 —[o]⟶K2MnO4 +H2O
K2MnO4 +2CO2 —–⟶ 2KnnO4+MnO2+ 2 K2CO3
K2MnO4 —-Cl2-⟶2KMnO4 +2HCl
USES
Antiseptic , antinfective , disinfectant , it is used in titrmetric analysis when it also act as self indicator.
Properties :-
Physical:-
It occurs as dark purple or almost black prismatic crystals or gramilar powder.
Odourless, almost opaque has blue metallic lusture
It is sweet with astringent after .It is stable in air , it decomposes when heated to 240°c with evolution of oxygen.
It is soluble in water , more soluble in boiling water. It is decomposed alcohol , also by some acids with liburation of oxygen.
Chemical :-
It is strong oxidizing agent . It oxidize iodides , to iodates [alkaline or neutralsolution]
In acidic solution , iodine i.e. liberated from iodides.
With HCL , cl2 is liberated
It oxidizes, sulphides to free Sulphur , ferrous to ferric salts and nitrites to nitrates,
KMnO4+ H2O+KI ⟶ 2MnO4 +KOH+KIO3
2KMnO4 +3H2SO4+10KI⟶2MnSO4+KOH+KIO3 +3H2O+6K2So4
BORIC ACID (H3 BO3)
METHODS OF PREPARATION
It may be prepared by decomposition of Borax.
Borax is treated with cone. H2SO4 and H2O insoluble sodium sulphate is also obtained which is separated by filteration and filtrate on crystalisation gives boric acid.
Na2B4O + H2SO4 + 3H2O ⟶ 4H3BO3 + Na2SO4 +H2
(Borax) (Boric acid) (insoluble sodium sulphate)
From natural sources
It comes out with jets of steams c/d SOFFIONI from the ground in certain parts of TUSCANY the condensed steam is concentrated by its own heat cooled and crystallized Boric acid is obtained.
PHYSICAL PROPERTIES
It occurs as odourless , colourless,transparent crystals , white granules or powder.
It is gressy to touch and sweetish after taste.
It is slightly soluble in water and more soluble in glycerine.
CHEMICAL
It is a week acid and solution gives slightly red cp;our with litmus.
Heating of Boric acid to certain temperature produces different dehydration products
For e.g:- when heated to 100° it causes the loss of 1 water molecule produce metaboric acid.
Heating at low 60° causes a further loss of water molecule to produce tetraboric acid and heating further to higher temperature produces anhydride of Boric acid c/d BORONTRIOXIDE
H3BO3 —–∆ 100°⟶ H BO2 —–∆ 60°⟶ H2B7 —–∆ ⟶2B2O3
(-1H2O) metaboric acid tetraboric acid boron trioxide
A mixture of boric acid and sthylalcohol burns with a green flame due to formation of ethyle borate
The treatment of Boric acid with equimolar (same molar quantity ) amount of glycerine at 140° to 100°c produces a compound boroglycerine glycerite which is used as suppository base.
USES
Boric acid is a weak bacteriostatic, fungistatic and antiseptic properties if is used as an antimicrobial eyewash solution which is also used as a part of a buffer system.
It also used as insecticide for cockroaches.
HYDROGEN PEROXIDE (H2O2)
It is an aquous solution containing 26-28% w/w H2O2 it may contain suitable stabilizing agent.
PREPARATION
It may be prepared by treating Barium peroxide with cold dilute Sulphuric acid.
BaO2 + H2SO4 ⟶ H2O2 +BaSO4 (Barium Sulphate)
It can also be prepaed by passing CO2 to barium peroxide.
BaO2 +CO2 +H2O ⟶ H2O2 +BaCO3
It may also be prepared by decomposition of sodium peroxide using dilute Sulphuric acid
Na2O2 + H2SO4 ⟶ H2O2 + Na2SO4
It can also be prepared by electrolysis of 50% sulphuric acid followed by vacuum distillation.
USES
Used as disinfectant
Anti infactive
As a mild antiseptic to clean wounds. It has oxidizing action.
H2O2 ear drops are used to remove wax.
It is also used as bleaching and oxidizing agent
PROPERTIES
PHYSICAL
H2O2 is a clear colouless odourless or having odour resembling oxone. Bitter taste , acidic to litmus, caustic to skin water soluble either soluble and decomposed by many organic solvents.
H2O2 solution gradually deteriorates on standing therefore, some stablising agent is added . it decomposes by many oxidizing or reducing substances and alkalies . it is unstable on prolonged exposure to light and heat . it is to be stored at 18-15° protected from light in partially filled containers using some stabilizing agents which may be sulphuric acid, phosphoric acid , some preventive or complexing agent.
It decomposes to water and oxygen rapidly in alkaline solution when heated to 100° or in presence of iron or magnese ions
2H2O2 ⟶2 H2O+O2
H2O2 is a strong oxidizing agent . it oxidizes sodium sulphide to sodium sulphate . Ferrous sulphide to ferric sulphate lead sulphide to lead sulphate.
PbS+4H2O2 ⟶ PbSO4 + 4H2O
It liberates iodine from potassium iodide solution
2KI +H2O2 ⟶ I2+ 2KOH
Also behaves as reducing agent . it reduses KMnO4
KMnO4 +H2SO4 + 5H2O2 ⟶ K2SO4+ MnSO4+8H2O+5O2
It reduces ozone to oxygen, chlorine to chloride
Cl2+H2O2 ⟶ 2Hcl +O2
It is slightly acidic in nature and dilute solution is neutral towards litmus.
It reacts with alkalies for e.g- sodium hydroxide to give peroxide NaOM+H2O2 ⟶ Na2O2 +2H2O
When Hydrogen prroxide is added to acidified aquous solution of potassiumchloromate containing ether. A deep colour is obtained which is due to formation of “ perchromic acid .“
PRINCIPLE : OXIDATION REDUCTION TITRATION
Titration : standard solution of KMnO4
Indicator : KMnO4 Self indicator.
Sample is dilute with H2O and treated with H2SO4. This mixture is titrated with standard solution of KMnO4 . till a permanent pink colour is obtained.
Eq factor:- each ml od 0.02 molar KMnO4 = 0.0017g of O2
2MnO4- + 6M+ +5H2O2 ⟶ 2Mn2+ + 8H2O +5O2
CHLORINATED LIME
Other name (BLEACHING POWDER)
Formula : CaOCl2 · H2O
(Indian pharmacopeia)
I>P limit : It contains not less than 30% w/w of available chlorine.
PREPARATION
It is prepared by passing chlorine gas over dried slaked lime(CaO) Ca(OH)2
Ca(OH)2 Cl2⟶ CaOCl2 · H2O
Ca(OH)2 + HCl ⟶ CaOCl2 +H2O
PHYSICAL PROPERTIES
White , solid, dry or white dull poeder strong smell of chlorine
It has little solubility in water and Alcohol on exposure to air it becomes moist and decomposes to give hypochlorous acid. It absorbs CO2 and evolves chlorine. It is stored in dry and well closed container.
Aqueous solutions are strongly alkaline.
CHEMICAL PROPERTIES
When treated with dilute acid it liberates Hypochlorous acid which behaves as an oxidizing bleaching agent
2CaCCl2 + H2SO4 ⟶ HClO + CaCl2 + CaSO4
HClO ⟶ HCl+[O]
On treatment with excess of dilute acid chlorine is liberated.
With CO2 also chlotinated lime liberates chlorine
CaOCl2 +CO2 ⟶ CaCO3 + Cl2
AUTO OXIDATION/ SELF OXIDATION of chlorinated lime gives calcium chloride.
USES
Chlorinated lime has bacterial actions . is is used as bleaching agent.
It is used as sisinfactant for drinking water and swimming pool.
ASSAY
Principle:- IODOMETRIC TITRATION
TITRANT :- Standard solution of sodiumthio sulphate
INDICATOR :- Starch.
END POINT :- Disappearance of blue colour.
Theory:-
An aqueous suspension of sample is treated with acetic acid in the presence of KI (Potassium Iodide)
Chlorine is evolve which displaces an equivalent amount of iodine from potassium iodide (KI) the liberated iodine is titrated with standard solution of sodium thiosulphate using starch as an indicator.
END POINT: disappearance of blue colour.
CaOCl2 +CH3COOH ⟶ Ca(CH3COOH)2 +HCl + HOCl
HCl +HOCl ⟶ H2O+Cl2
Cl2+ KI ⟶ 2KCl +I2
Na2S2O3 +I2 ⟶ Na2S4O6
(sodium tetra thiorate)
EMETICS
Emetics are the agents which when taken orally or by injection induce/evoke the vomiting.
Mechanism of action– It generally act by two ways :-
- By stimulation of chemoreceptor trigger zone(CTZ) located in the area of postrema medulla oblongata in brain.
- By local irritating effect on GIT. E.g. copper sulphate, sodium chloride, zinc sulphate, antimony potassium tartrate.
Contraindications- Emetics should not be used in conditions like:-
- Significant CNS depression and shock.
- Unconscious or semi-consciousness or coma situations.
- Patient with severe heart disease.
- In tuberculosis, anemia and advanced pregnancy.
- Poisoning caused by corrosive or petroleum product.
Applications:-
1.Mechanical antidote
2.Sometimes emetics are added in cough preparations in low dose to stimulate the flow of respiratory tract.
Some of the common Emetics are :-
COPPER SULPHATE
Mol. Formula- CuSo4.5H2O
Mol. Wt.- 249.7gm
Synonyms:- Blue vitriol, cupric sulphate
Standard → it contains not less than 98.5% and not more than 100.5% calculated with reference to the dried substance at 250ׄ c.
Method of preparation–
2Cu+S+3O2 → CuSo4+2CuO
↓dil. H2SO4
2CuSO4 +H2O
Physical properties-
- It exists in the form of deep blue crystal of pentahydrate available in the form of granules or powder.
- It shows effervescence in dry air slowly.
- It is soluble in water, very soluble in boiling water and insoluble in alcohol.
Chemical properties-
- On heating at 100ׄ c it loses the water molecules and form anhydrous solution.
CuSo4.5H2O —100 ͦc—→ CuSo4.3H2O+2H2O —100 ͦc—→ CuSo4.2H2O+H2O
↓ 200 ͦ c
CuSO4[anhydrous salt]
- At very high temperature, it decomposes to cupric oxide and Sulphur dioxide gas.
2CuSo4→2CuO+So2+O2
Assay :- The principle involved in assay of copper sulphate is oxidation reduction reaction. This reaction is based on the instability of CUI formed in the reaction of copper sulphate with potassium iodide, which decomposes to give cuprous iodide with the liberation of free iodine.
2CuSo4+4KI→2CuI2+2K2SO4
2CuI2→Cu2I2+I2
2gm of potassium thiocyanate is then added and titrated until the blue colour disappears.
I2+2Na2S2O3→Na2S4O6+2NaI
Cu2I2+KCNS→2CuNS+2KI
Each 1 ml of 0.1N sodium thiosulphate ≡0.02497gm of CuSO4.5H2O
Identification test:- A 5% w/v sample solution of cupric sulphate is tested for copper and sulphate.
Test for purity:- 1.Chlorides not more than 100 ppm.
2.Iron not more than 10 ppm.
3.Lead not more than 50 ppm.
4.Loss on drying not more than 33-36.5%
Uses– 1. As emetics, but in large doses, it is corrosive in nature
2.chemical antidote in phosphorous poisoning
3.Externally it is astringent and fungicidal
4.It is ingredient of benedicts and Fehling’s reagent
Sodium potassium Tartrate (C4H4KNaO6 ∙ 4H2O)
Synonyms :- Rochelle salt
Seignette salt
PREPARATION :-
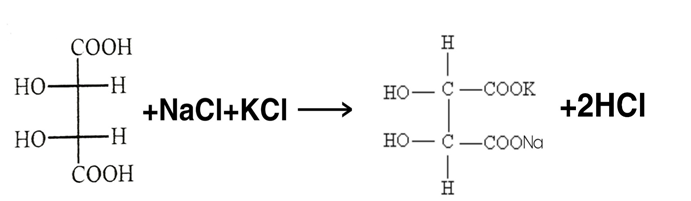
Tartaric acid Sodium potassium Tartrate
Physical properties-
It is crystalline powder, odourless freely soluble in water and insoluble in alcohol.
Chemical properties-
On heating, it gives odour of burning sugar.
C4H4O6KNa.4H2O+5O2→K2CO3+Na2CO3+8H2O+6CO2
Identification test:- As chemical properties
Storage:- Air tight container
Dose:- 10gm to adult
Uses:- 1. Saline cathartic.
2.Depending upon the dose, it is also used as mild laxative.
3. It can also be used as diuretic and urinary alkalizer.
4. It is used as food additive as a stabilizer in meat and cheese
Products.
5.It is an ingredient of compound effervescent powder.
HAEMATINICS
These are the agents which are required for the formation of blood cells and also used in the treatment of anemia. Eg. Ferrous sulphate, ferrous glueonate.
Anemia is decreased capacity of RBCs to carry oxygen to the tissues. It occurs when the balance between production and destruction of RBCs is disturbed.The disturbance can occur due to
- Blood cells
- Impaired red blood cell formation due to deficiency of essential factors i.e. Iron, vitamin B12, folic acid or bone marrow depression.
- Increased destruction of RBCs ( Haemolytic anemia )
Ferrous sulphate(FeSO4.7H2O)
Method of preparation
Fe+H2SO4→FeSO4+H2↑
Physical properties:-
It is odourless, bluish green crystal or powder, metallic taste and astringent. It is completely soluble in water and insoluble in alcohol.
Chemical properties:-
- On heating it decomposes to ferric oxide, sulphur dioxide and sulphur trioxide.
2FeSO4→Fe2O3+SO2+SO3
- It reduces to the salt of silver and gold to their corresponding metals.
Ag++Fe2+→Ag+Fe3+
Au3++3Fe2+→Au+3Fe3+
Storage:-Air tight containers
Assay:- principle-Redox titration
An accurately weighted 1g powder is dissolved in 20ml of dilute HCL. Then this solution is titrated against 0.1NKMn
10FeSO4+2KMnO4+8H2SO4→K2SO4+2MnSO4+5Fe2(SO4)3+H2O
Here KMnO4 solution acting as self indicator.
Each 1ml of 0.1NKMnO4≡0.0291gm of Feso4
Dose:- It is given in a dose of 300-400 mg daily.
Uses:– 1. It is used as hematinic.
2. It is used in the treatment of anemia caused by iron deficiency.
3. It also posses disinfectant property.
4. It is used as an insecticide in agriculture.
Caution:– 1.Excessive consumption by children may cause GIT irritation Or shock.
2.It may cause discoloration of teeth in contact.
Poison and Antidotes
POISONS
A poison may be defined as any substance administered in whatever way ( by mouth, injection , inhalation , skin ) produces ill health, diseases or death.
Self medication is a major cause of drug poisoning.
Poisoning can be classified as :-
(A) Intentional poisoning – taking substances without intention of causing harm to self. E.g. :- Suicide
(B) Unintentional poisoning – Taking substances without knowing its toxic effects. E.g. :- Accidental
Signs and symptoms of poisoning:-
- Reduced breathing rate
- Nausea
- Vomiting and Diarrhoea
- Alteration in heart rate
- Muscle cramps
- Partial Consciousness.
(C) Heavy Metal poisoning –
This poisoning occurs due to intake of salt of As, Pb , Hg or Fe resulting into the toxic effect
Treatment :- Activated charcoal given initially to absorb heavy metal ↓ followed by emetics to eliminate any poison left in the stomach.
(D) Cyanide poisoning :-
This poisoning may occur by inhalation of fumes of hydrocyanic acid (HCN) or inorganic cyanide salt.
Consumption of 300 mg of KCN may cause death.
Treatment :- Sodium nitrite and Sodium thiosulphate injections as an antidotes.
ANTIDOTES
Antidotes are the substances which react specifically without the ingested poison or toxic substances or with potent drugs in case of overdose.
CLASSIFICATION :-
According to their mechanism of actions, they are classified as:
Physiological Antidotes –
They are antagonists i.e. produce the effect opposite to that of the poison
E.g.:-
Antagonists and Physostigmine are two antidotes for each other.
Sodium nitrite in CN- poisoning.
(A) Chemical Antidotes –
They react by combining with the poison and change its chemical nature by converting poison into inactive compounds.
(B) Mechanical Antidotes E.g. – Sodium thiosulphate convert toxic cyanide into non-toxic thiocyanate.
EDTA as chelating agent for heavy metal poisoning. They act by preventing the absorption of poison into the body or expel out the poison by emesis
E.g. :- Activated charcoal absorbs the poison to absorption into intestinal wall.
SODIUM THIOSULPHATE [Na2S2O2]
Synonyms :- Sodium hyposulfite, Anti-chloral
Preparation– Prepared by boiling Sodium Sulphite Na2S2O2 with Sulphur (S)
Na2SO3 + S —∆⟶ Na2S2O3 (Sodium thiosulfate)
By reacting sodium hydroxide with Sulphur.
6NaOH + 4S ⟶ Na2S2O3 + 2 Na2S + 3H2O
Physical properties –
- Large, prismatic crystalline powder
- Effervesces in dry air
- Practically soluble in H2O and insoluble in alcohol
- Melting point- 50°c
Chemical properties –
Decomposition of aq. Solution as Sodium thiosulfate Sodium sulfate Sodium
4Na2S2O3 ⟶ 3Na2SO4+ Na2S5 ⟶ Na2S + 4S
Barium chloride reacts without to give white ppt. of barium thiosulphate
Na2S2O3 + BaCl2 ⟶ BaS2O3↓ + 2NaCl
Mechanism of antidotes :-
Slow infusion of sodium thiosulfate
↓
react with CN- in the blood
↓
Convert CN- to SCN- (thiocyanate)
↓
SCN- excreted out from the body by kidney.
Na2S2O3 + CN- ⟶ SCN- + Na2S2O3
(Active cyanide) (inactive thiocyanate)
Uses :-
*Used as antidote in cyanide poisoning as IV
*Effective antidote in Pb, Hg and iodine poisoning.
*Use as antioxidant for solution containing iodides
*Standard titrant in Iodimetric analysis.
Dose = 0.3 – 1g (10ml) administrated by intra muscular and Intra venous route.
Assay :-
The assay of Na2S2O3 is based on iodometric titrations.
In this titration, solution of Na2S2O3 is titrated with Iodine directly using starch solution as an indicator.
Excess I2 reacts without starch to give blue colour (end point).
Iodometric Reactions involved :-
2S2O3– → S4O62- + 2e– (oxidation)
I2+2e– → 2I– (Reduction)
2S2O32- ≡ I2
Over all reaction:-
2Na2S2O3 + I2→Na2S4O6 + 2NaI
Equivalent factor:-
I2 ≡ 2Na2S2O3
100ml 1M I2 ≡ 2*248.2g Na2S2O3
1ml 0.05M I2 ≡ 0.02482g Na2S2O3
ACTIVATED CHARCOL :- It is a dark grey residue consisting of carbon and any remaining ash obtained by removing water and other volatile constituent.
Preparation:-
Burning of wood in absence of air
↓
Residue obtained consists of pure carbon
↓
(Activation of charcoal )
Absorptive powder of charcoal increased by treating it with various substance such as steam, CO2, ZnCl2 at high temperature 500-1000∙c
↓
Charcoal activation results in increase in total surface area
Properties:-
(Fine, black, odorless powder)
Insoluble in H2O and other organic solvent.
Uses:-
- Emergency antidotes in many forms of poisoning high preventing absorption of poison in intestinal tract.
- Used as protective and adsorbent
- Due to its high surface area, it is used as a fitter acid
- Disinfectant in wounds
- Constituent for gum powder
- Used in overdose of aspirin
SODIUM NITRATE (NaNO2)
Synonyms:-Nitrous acid sodium salt or Etinitrit
Preparation:- By adding heating sodium nitrate
2NaNO3 → 2NaNO2 + O2
(Sodium nitrate)
By heating sodium nitrate with lead
NaNO3+ Pb → NaNO2+ PbO
Properties:-
- Yellow and white crystalline powder.
- Freely soluble in H2O and less soluble in alcohol.
- Odorless
- On exposure to air, it readily forms sodium nitrate.
Mechanism of antidotes action:-
Injection of NaNO2 causes oxidation of ferrous ion of haemoglobin (Fe2+) into ferric ion (Fe3+) of methemoglobin
↓
Methemoglobin combines with serum cyanide and produce cyano-meth haemoglobin.
↓
Prevention of harmful of CN–
HB (Fe3+) NaNO2 → MeHB (Fe3+)
Methemoglobin
MeHB(Fe3+)+CN– → MeHB(Fe3+)CN– (Inactive cyanide ion)
Cyano-meth haemoglobin
Uses:-
Used as antidote in cyanide poisoning.
Vasodilator action due to nitrite ions relax the smooth muscle of blood vessels.
Anti – rust solution : to prevent the rusting of surgical instruments
Doses of injection :- 10-15 ml of 3% solution I.V.
Expectorants
These are the agents which enhance the secretion of sputum from trachea, bronchi or lungs and hence they are used in the treatment of cough.
Classification of Expectorants –
Based on their mechanism of action, expectorants are categorized into two types:-
- Sedative expectorants
- Stimulant expectorants
Sedative expectorants:- These are stomach irritant which are able to produce their effect through stimulation of gastric reflux. For example bitter drugs such as ipecac, senega Indian squill and compounds such as antimony potassium, tartarate, ammonium chloride, sodium citrate, potassium iodide, etc.
Stimulant expectorants:- Expectorant which bring about stimulation of secretory cells of respiratory tract directly or indirectly since these drugs stimulate secretion, more fluid gets produced in respiratory tract and hence sputum is diluted. Eg: eucalyptus, lemon, etc.
Mechanism of action:–
They act upon respiratory tract in two different ways:
- By decreasing the viscosity of bronchial secretion, it facilitates its easy removal by coughing.
- By increasing the amount of respiratory tract fluids, demulcent action is exerted over dry mucosal linings, their relieving the unproductive cough.
POTASSIUM IODIDE (KI)
Methods of preparation : It can be prepared by two different methods :
- Laboratory method : In laboratory, it can be easily prepared by treating slight excess of iodine with a lot aqueous solution of potassium hydroxide. The pale yellow solution obtained is evaporated to dryness and the residue (potassium iodide) is heated with charcoal to reduce iodate to iodide.
6KOH + 3I2 ——> KIO3 +5KI +3H2O
KIO3 + 3C ——> KI + 3CO
2. Industrial method : On commercial scale, it can be prepared by using potassium carbonate and iron fillings are agitated in the iodine solution to form ferroferric iodide (FeI2.FeI3) which on further boiling with concentrated solution of potassium carbonate gives potassium iodide.
4Fe+I2→2FeI2.FeI3
FeI2FeI3 + 4K2CO3 → 8KI + FeO.Fe2O3 + 4CO2
Physical properties:-
- Cubic crystals
- white granular powder
- hygroscopic in nature
- saline in taste and slightly bitter.
- It is soluble in water, glycerin and alcohol on exposure to air, it becomes due to liberation of iodine.
Chemical properties:-
- With silver nitrate, it gives yellow precipitate of silver iodine.
KI + AgNO3 → AgI (yellow)+KNO3
- Iodide ion gets easily oxidized to iodine when treated with oxidizing agents like chlorine, copper, nitric acid etc.
2I–→ I2 +2e–
- Iodine readily get dissolved in aq. solution of KI, forming a dark brown solution of potassium tri-iodide.
KI+I2 ↔ KI3
Uses:–
- As expectorant in dose of 300 mg 4 times a day.
- It acts as a source of iodine and potassium simultaneously.
- It is used in prophylaxis and treatment of goiter.
- It is saline diuretics.
- It is also used as antifungal agent in veterinary practices.
AMMONIUM CHLORIDE (NH4Cl)
Synonym:- salmiac, Amchlor, ammonium nitrate
Method of preparation:-
- It is prepared by neutralizing HCl with ammonia
NH3+HCl → NH4Cl
The resulting solution of ammonium chloride is evaporated to dryness.
- It is also prepared by treating ammonium sulphate with sodium chloride.
2NaCl+ (NH4)2SO4→2NH3+2NCl+Na2SO4
2NH3+2HCl→2NH4Cl
Physical properties:-
It is a white fine crystalline powder. It is odorless and has cooling saline taste, hygroscopic in nature, freely soluble in water but slightly soluble in alcohol. Its 0.8% w/v solution is isotonic with serum.
Chemical properties:-
In its vapour form, it dissociates in ammonia and HCl.
NH4HCl ↔ NH3+HCl
Assay:-
It is assayed by acid base titration. The neutral formaldehyde solution is added so that NH4HCl will be converted to methanilimine and HCl. The liberated acid is titrated with 0.1 N NaOH using phenolphthalein as an indicator.
NH4Cl+HCHO → HCH=NH+HCl+H2O
HCl+NaOH→NaCl+H2O
Each 1 ml of 0.1 N NaOH ≡ 0.05349 g of ammonium chloride.
Dose:- 3-6 gm daily in divided dose
Uses:-
- As expectorant: It is used as an ingredient in expectorant cough mixtures in doses of 300 mg to 1 gm.
- As diuretics: It is given for its diuretics actions especially to help the excretion of over dosage of basic drugs such as amphetamine and in the treatment of lead poisoning by increasing of its excretion.
- As systemic acidifier: It is helpful in producing mild acidosis.

0 Comments